
Navigating the Labyrinth: ISO 13485 Training and the Art of Medical Device Quality
Navigating the Labyrinth: ISO 13485 Training and the Art of Medical Device Quality
ISO 13485 Training Courses in USA
The medical device industry operates within a complex ecosystem of regulations and stringent quality requirements. ISO 13485, the internationally recognized standard for quality management systems specific to medical devices, isn't just a hurdle to overcome; it's a compass guiding manufacturers towards patient safety and regulatory compliance. But simply possessing the certificate is insufficient. True mastery of ISO 13485 requires a deep understanding cultivated through targeted, innovative training.
ISO 13485 Training Courses in USA
Beyond Regulatory Checkboxes: Cultivating a Culture of Vigilance
Traditional ISO 13485 training often focuses on ticking boxes and memorizing clauses. While compliance is vital, it can lead to a superficial understanding, missing the standard's core intent. Modern training must transcend this, focusing on:
Risk Management as a Core Principle: ISO 13485 is intrinsically linked to risk management. Training should emphasize how to identify, evaluate, and control risks throughout the medical device lifecycle, from design to post-market surveillance.
The Patient-Centric Approach: Reinforcing that every process and decision ultimately impacts patient safety. Training should instill a deep sense of responsibility and ethical awareness.
Understanding the Regulatory Landscape: The medical device industry is subject to diverse and evolving regulations. Training must provide a comprehensive overview of relevant regulatory requirements, including those specific to target markets.
Process Validation and Control: Medical devices are complex, and their manufacturing processes must be rigorously validated and controlled. Training should equip participants with the skills to effectively implement and maintain these controls.
Post-Market Surveillance and Vigilance: Understanding that a device's life cycle continues after sales, and that continuous improvement through post market data is fundamental. Training should equip participants to understand and implement these processes.
Transforming Learning: Engaging and Effective Techniques
Effective ISO 13485 training goes beyond passive lectures. It embraces interactive and engaging techniques, such as:
Simulation-Based Learning: Replicating real-world scenarios to provide hands-on experience in applying ISO 13485 principles.
Case Studies and Role-Playing: Analyzing real-life incidents and engaging in role-playing exercises to develop critical thinking and problem-solving skills.
Interactive Workshops and Group Discussions: Fostering collaboration and knowledge sharing among participants.
Utilizing Digital Platforms and Virtual Reality: Creating immersive learning experiences that enhance understanding and retention.
Tailored training: Creating training programs that are specific to the customers devices and processes.
The Ripple Effect: Benefits Beyond Compliance
Investing in robust ISO 13485 training yields significant benefits beyond regulatory compliance. It fosters:
Enhanced Patient Safety: By prioritizing quality and risk management, organizations can minimize the risk of adverse events and improve patient outcomes.
Improved Product Quality and Reliability: Rigorous quality control processes ensure that medical devices consistently meet performance and safety requirements.
Increased Regulatory Compliance and Market Access: Demonstrating compliance with ISO 13485 facilitates regulatory approvals and expands market access.
Reduced Costs and Risks: Proactive risk management and process optimization can minimize the costs associated with recalls, rework, and regulatory penalties.
Enhanced Reputation and Brand Trust: A strong commitment to quality enhances an organization's reputation and builds trust with customers and stakeholders.
Cultivating a Culture of Excellence: A Continuous Journey
ISO 13485 training is not a one-time event; it's a continuous journey of learning and improvement. By fostering a culture of quality, organizations can ensure that they are not only meeting regulatory requirements but also delivering safe and effective medical devices that improve the lives of patients worldwide. It is turning the ISO 13485 standard from a burden, into a tool that improves the business.
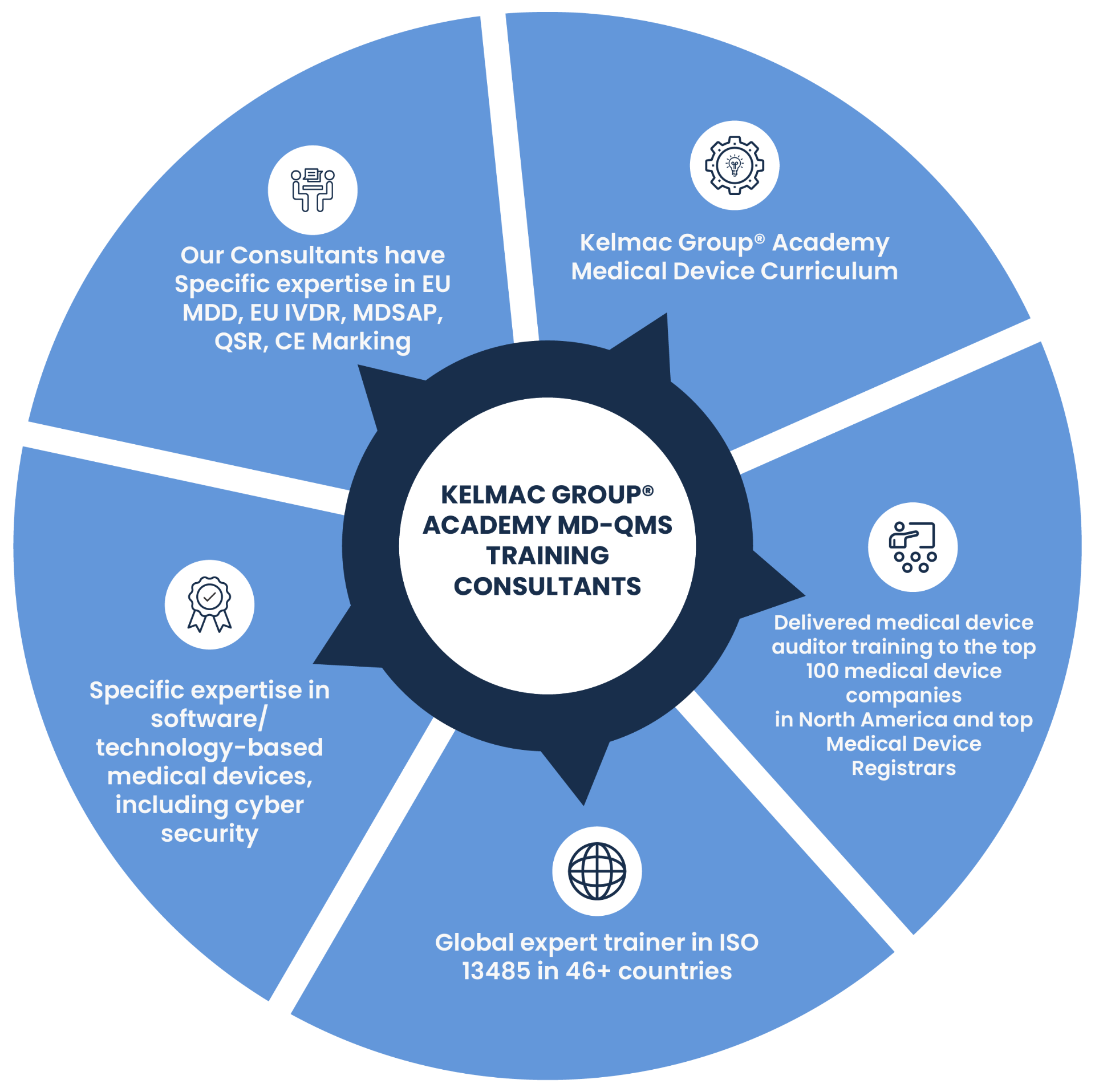
Kelmac Group offers ISO 13485:2016 Training Courses in the USA, including Fundamental, Internal Auditor, and Lead Auditor programs. These accredited courses are designed to equip professionals with essential knowledge of medical device quality management systems, from basic principles to advanced auditing skills. Flexible virtual in-house training options are available, ensuring your team achieves compliance and drives business success. Enroll now to enhance your expertise!